Biohybrid microswimmers exploit the swimming and navigation of a motile microorganism to target and deliver cargo molecules in a wide range of biomedical applications. Microalga-powered biohybrid microswimmers offer great potential compared to other motile microorganisms, considering their full biocompatibility, superior motility and phototactic abilities. Therefore, we have developed high-throughput methods to prepare microalga-powered biohybrid microswimmers that carry complex and functional cargoes, and we aim to target and localize them for various medical applications.
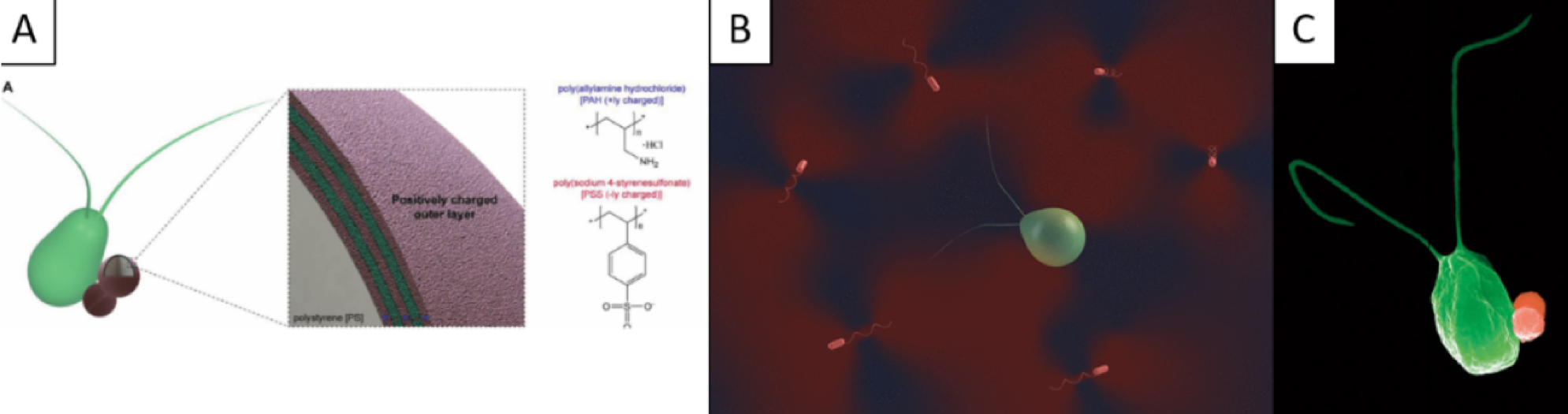
First, we designed and manufactured a biocompatible biohybrid microswimmer powered by a unicellular freshwater green microalga, Chlamydomonas reinhardti (C. reinhardtii) and polyelectrolyte-functionalized magnetic spherical cargoes [ ]. We also showed their biocompatibility and external magnetic steering. Next, we investigated puller and pusher-type of microswimmers, where we showed that in a co-culture of C. reinhardtii algae with Escherichia coli (E. coli) bacteria [ ], we observed noticeable fluidic coupling deviations from the existing understanding of passive colloids mixed with bacterial suspensions previously studied in the literature.
We also developed magnetotactic algal microswimmers, by magnetizing microalgal cells with terbium [ ]. The magnetized algae were able to align to the field lines of an applied uniform magnetic field, guiding them to swim in a directional motion. Considering their non-cytotoxic nature and autofluorescence, magnetized microalgal biohybrids are promising candidates for magnetically steerable biohybrid microrobots. In a recent work, we reported a new biohybrid design strategy, where we non-covalently assembled a thin and soft uniform coating layer around C. reinhardtii [ ]. We used polymer-nanoparticle matrix as the synthetic component, reaching a manufacturing efficiency of approximately 90%. Furthermore, by conjugating the nanoparticles embedded in the thin coating with chemotherapeutic doxorubicin by a photocleavable linker, we reported on-demand delivery of drugs to tumor cells as a proof-of-concept biomedical demonstration.
